electron affinity of si|Silicon : iloilo • Janousek, Bruce K.; Brauman, John I. (1979), "Electron affinities", in Bowers, M. T. (ed.), Gas Phase Ion Chemistry, vol. 2, New York: Academic Press, p. 53.• . Tingnan ang higit pa poweredge R740 开机提示 UEFI0339: The Dual Inline Memory Module (DIMM) in the memory slot A1 is disabled because of initialization errors caused by uncorrectable memory errors, invalid configuration, .Harry Potter Clue - Rule discussion We love playing this game as a family but we are having somewhat of a debate tonight. For those that have played, the rules state you must leave a room each turn and can not return to that room on the same turn.
PH0 · The General Properties of Si, Ge, SiGe, SiO2 and Si3N4
PH1 · Silicon
PH2 · Electron affinity (data page)
PH3 · Electron affinity
PH4 · Electron Affinity Chart (Labeled Periodic table + List)
PH5 · Electron Affinity
PH6 · A7: Electron Affinities
PH7 · 7.5: Electron Affinities
PinayFlix VIP is a free Pinay porn site to watch Pinay scandal videos. Watch rare Filipina videos and other Asian amateur clips. You are about to enter a website that contains Adult Explicit Material.
electron affinity of si*******Electron affinity can be defined in two equivalent ways. First, as the energy that is released by adding an electron to an isolated gaseous atom. The second (reverse) definition is that electron affinity is the energy required to remove an electron from a singly charged gaseous negative ion. . Tingnan ang higit paThis page deals with the electron affinity as a property of isolated atoms or molecules (i.e. in the gas phase). Solid state . Tingnan ang higit pa
• Janousek, Bruce K.; Brauman, John I. (1979), "Electron affinities", in Bowers, M. T. (ed.), Gas Phase Ion Chemistry, vol. 2, New York: Academic Press, p. 53.• . Tingnan ang higit pa Ago 11, 2023
The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. X(g) + e → X (g) + energyThis differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on electron capture.This paper summarizes basic physical properties of Si, Ge, SiGe, SiO2 and Si3N4. It also lists several physical constants and conversion factors. The information is presented in .
The electron affinity is defined as the amount of energy released when an .To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. To recognize the inverse relationship of ionization energies . Unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gas atom. .
In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or molecule .The electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative ion. For example, when a neutral chlorine atom in the .26.11.2021 by Author. Electron Affinity and Electronegativity of Silicon. Electron Affinity of Silicon is 133.6 kJ/mol. Electronegativity of Silicon is 1.9. First Ionization Energy of .
electron affinity of si A charge density plot of four Si–C bilayers from an unterminated 4H–SiC slab against position in the [0001] direction. . Incomplete coverage both alters the surface dipole density affecting the extent to which electron affinity is changed compared to an unterminated surface, but also generates surface states that may be non-physically .
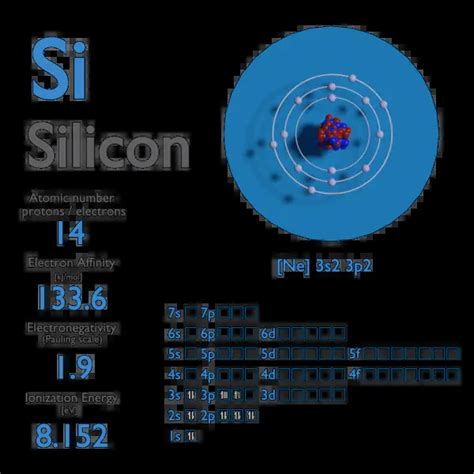
Electron affinity of Silicon is 133.6 kJ/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. X + e– → X– + energy Affinity = – ∆H.Effective electron masses m l: 0.98m o: Effective electron masses m t: 0.19m o: Effective hole masses m h: 0.49m o: Effective hole masses m lp: 0.16m o: Electron affinity: 4.05 eV: Lattice constant: 5.431 A: Optical phonon energy:Si: properties of free atoms. Silicon atoms have 14 electrons and the shell structure is 2.8.4. The ground state electron configuration of ground state gaseous neutral silicon is [ Ne ]. 3s2. 3p2 and the term symbol is 3P0. Schematic electronic configuration of silicon. The Kossel shell structure of silicon.The electronic affinity is amount of energy, that is released during the attachment of the electron to the neutral atom. As a result of such attachment, a negative ion (anion) is formed. Electron affinity is related to electronegativity of elements.Simply speaking, the greater the affinity of electrons, the more eagerly the atoms of a given element join .
Electron Affinities. Electron affinity, often abbreviated as EA, is the energy released when an electron is added to a valence shell of the atom. F (g) + e- -> F-(g) EA = -328 kJ/mol. [When an electron is added to an . Electron affinities are more difficult to measure than ionization energies. An atom of Silicon in the gas phase, for example, gives off energy when it gains an electron to form an ion of Silicon. Si + e – → Si – – ∆H = Affinity = 133.6 kJ/mol. Electron affinity is one of the most important parameters that guide chemical reactivity.The electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative ion. For example, when a neutral chlorine atom in the gaseous form picks up an electron to form a Cl- ion, it releases an energy of 349 kJ/mol or 3.6 eV/atom. It is said to have an electron affinity of -349 kJ/mol and this .

The electron affinity [EA] is the energy change for the process of adding an electron to a gaseous atom to form an anion (negative ion). X(g) +e− X−(g) EA1 (3.4.1) (3.4.1) X ( g) + e − X − ( g) EA 1. This process can be either endothermic or exothermic, depending on the element. The EA of some of the elements is given in Figure 3.4.6 3.4.
Photodetachment microscopy has been performed on a beam of 32S- ions. Analysing the electron images obtained, we find that the electron affinity measurements performed with the photodetachment microscope contain a small bias, due to the difference between the actual and assumed values of the applied electric field. Having a measure . The electron affinity ( EA E A) of an element E E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: E(g) +e− → E−(g) energy change=EA (4.5.1) (4.5.1) E ( g) + e − → E ( g) − energy change= E A. Unlike ionization energies, which are always positive for a neutral atom because energy is .
electron affinity of si Silicon slight. The work function for Si (100) was determined to be about 4.85 eV for both intrinsic and p- or n-doped single crystal samples.[3‐5] The work function for the p-type Si (111) surface was determined to be 4.6 eV from UPS[6]. As further checking, we have confirmed with UPS characterization of the clean Si
Electron Affinity. The electron affinity (EA) of an element E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: [latex]E_{(g)}+e^- \rightarrow E^-_{(g)} \;\;\; \text{energy change=}EA \label{7.5.1}[/latex] Unlike ionization energies, which are always positive for a neutral atom because energy is required to .A7: Electron Affinities. The electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or molecule in the gaseous state to form a negative ion. X(g) +e− → X−(g) + energy (A7.1) (A7.1) X ( g) + e − → X ( g) − + e n e r g y. This negative electron affinity (NEA) influences the efficiency and current density of an electron source. In contrast where species more electronegative than carbon, . Si and Ge atoms at 67% coverage sit above the C site and co-ordinate fully, twice with carbon and adjacent crystallogen atoms. Relatively modest NEAs for all coverages . Further reduction in electron affinity deteriorates cell efficiency. This can be theoretically confirmed by the band-bending diagram of the ZnO/Si junction as shown in Figure 4. Since the electron affinity of Si is ~4.05 eV, the electron affinity of ZnO below this value results in formation of a spike in the conduction band of the n-ZnO region.Enter an electron affinity value or range in eV: If desired, enter a formula to restrict the search: Allow elements not specified in formula. Allow more atoms of elements in formula than specified. Select the desired units for thermodynamic data: SI calorie-based; Select the desired type(s) of data:Silicon Effective electron masses m l: 0.98m o: Effective electron masses m t: 0.19m o: Effective hole masses m h: 0.49m o: Effective hole masses m lp: 0.16m o: Electron affinity: 4.05 eV: Lattice constant: 5.431 A: Optical phonon energy:
A subreddit for the Total War strategy game series, made by Creative Assembly. Discussions, strategies, stories, crude cave-drawings, and more for Medieval 2, Empire, Shogun 2, Rome 2, Attila, Thrones of Britannia, Warhammer, Three Kingdoms, Troy, Pharaoh and others.
electron affinity of si|Silicon